WBJEE - CHEMISTRY
- Number of Questions : 20
- Time : 30 Minutes
- No negative marking.
Start
Congratulations - you have completed WBJEE - CHEMISTRY.
You scored %%SCORE%% out of %%TOTAL%%.
Your performance has been rated as %%RATING%%
Your answers are highlighted below.
Question 1 |
The amount of the heat released when 20 ml 0.5 M NaOH is mixed with 100 ml 0.1 M HCl is x kJ. The heat of neutralization is
A | – 100 x kJ/mol |
B | – 50 x kJ/mol |
C | + 100 x kJ/mol |
D | +50 x kJ/mol |
Question 2 |
If the molecular wt. of Na2S2O3 and I2 are M1 and M2 respectively, then what will be the equivalent wt. of Na2S2O3 and I2 in the following reaction?
A | M1 , M2 |
B | M1 , M2/2 |
C | 2M1 , M2 |
D | M1 , 2M2 |
Question 3 |
An electric current is passed through an aqueous solution of a mixture of alanine (isoelectric point 6.0) glutamic acid (3.2) and arginine (10.7) buffered at pH 6. What is the fate of the three acids?
A | Glutamic acid migrates to anode at pH 6. Arginine is present as a cation and migrates to the cathode. Alanine in a dipolar
ion remains uniformly distributed in solution. |
B | Glutamic acid migrates to cathode and others remain uniformly distributed in solution |
C | All three remain uniformly distributed in solution |
D | All three move to cathode |
Question 4 |
2 gm of metal carbonate is neutralized completely by 100 ml of 0.1 (N) HCl. The equivalent weight of metal carbonate is
A | 50 |
B | 100 |
C | 150 |
D | 200 |
Question 5 |
The electronic transitions from n = 2 to n = 1 will produce shortest wavelength in (where n = principal quantum state)
A | Li+2 |
B | He+ |
C | H |
D | H+ |
Question 6 |
The ease of dehydrohalogenation of alkyl halide with alcoholic KOH is
A | 3° < 2° < 1° |
B | 3° > 2° > 1° |
C | 3° < 2° > 1° |
D | 3° > 2° < 1° |
Question 7 |
In the following electron – dot structure, calculate the formal charge from left to right nitrogen atom
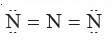
A | – 1, –1, + 1 |
B | – 1, +1, – 1 |
C | + 1, –1, – 1 |
D | + 1, – 1, + 1 |
Question 8 |
The ozone layer forms naturally by
A | the interaction of CFC with oxygen |
B | the interaction of UV radiation with oxygen |
C | the interaction of IR radiation with oxygen |
D | the interaction of oxygen and water vapour |
Question 9 |
The standard reduction potential E° for half reations are
Zn = Zn+2 + Ze E° = +0.76 V
Fe = Fe+2 + Ze E° = + 0.41 V
The EMF of hte cell reaction
Fe+2 + Zn = Zn+2 + Fe is
A | -0.35 V |
B | + 0.35 |
C | +1.17 V |
D | – 1.17 V |
Question 10 |
An element belongs to Group 15 and third period of the periodic table. Its electonic configuration will be
A | 1s22s22p3 |
B | 1s22s22p4 |
C | 1s22s22p63s23p3 |
D | 1s22s22p63s23p2 |
Question 11 |
The energy of an electron in first Bohr orbit of H – atom is – 13.6 eV. The possible energy value of electron in the excited state of Li2+ is
A | – 122.4 eV |
B | – 50.6 eV |
C | – 30.6 eV |
D | 13.6 eV |
Question 12 |
Which one of the following is paramagnetic?
A | N2 |
B | NO |
C | CO |
D | O3 |
Question 13 |
Among the alkenes which one produces tertiary bytyl alcohol on acid hydration
A | CH3 – CH2 – CH = CH2 |
B | CH3 – CH = CH – CH3 |
C | (CH3)2C = CH2 |
D | CH3 – CH = CH2 |
Question 14 |
The representation of the ground state electronic configuration of He by box – diagram as ↑ ↑ is wrong because it violates
A | Hysenberg’s Uncertainty Principle |
B | Bohr’s Quantization Theory of Angular Momenta |
C | Pauli Exclusion Principle |
D | Hund’s Rule |
Question 15 |
A radioactive atom XYM emits two α particles and one β particle successively. The number of neutrons in the nucleus of the product will be
A | X – 4 – Y |
B | X – Y – 5 |
C | X – Y – 3 |
D | X – Y – 6 |
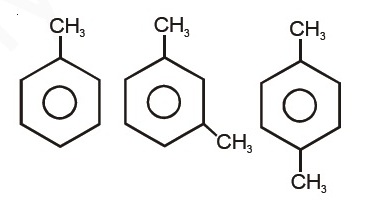
Question 16 |
A | II = III ≈ I |
B | II > III > I |
C | III > II > I |
D | I = III > II |
Question 17 |
The correct order of decreasing acidity of nitrophenols will be
A | m-Nitrophenol > p-Nitrophenol > o-Nitrophenol
|
B | p-Nitrophenol > m- Nitrophenol > o-Nitrophenol |
C | o-Nitrophenol > m- Nitrophenol > p-Nitrophenol |
D | p-Nitrophenol > o-nitrophenol > m-Nitrophenol |
Question 18 |
Which one of the following is not true at room temperature and pressure
A | P4O10 is a white solid |
B | SO2 is a colourless gas |
C | SO3 is a colourless gas |
D | NO2 is a brown gas |
Question 19 |
Two aromatic compounds having formula C7H8O which are easily identifiable by FeCl3 solution test (violet colouration) are
A | o- cresol and benzyl alcohol |
B | m-cresol and p-cresol |
C | o-cresol and p-cresol |
D | methyl phenyl ether and benzyl alcohol |
Question 20 |
Which one of the following has the lowest ionization energy?
A | 1s22s22p6 |
B | 1s22s22p63s1 |
C | 1s22s22p5 |
D | 1s22s22p3 |
Once you are finished, click the button below. Any items you have not completed will be marked incorrect.
Get Results
There are 20 questions to complete.
← |
List |
→ |
Return
Shaded items are complete.
| 1 | 2 | 3 | 4 | 5 |
| 6 | 7 | 8 | 9 | 10 |
| 11 | 12 | 13 | 14 | 15 |
| 16 | 17 | 18 | 19 | 20 |
| End |
Return
You have completed
questions
question
Your score is
Correct
Wrong
Partial-Credit
You have not finished your quiz. If you leave this page, your progress will be lost.
Correct Answer
You Selected
Not Attempted
Final Score on Quiz
Attempted Questions Correct
Attempted Questions Wrong
Questions Not Attempted
Total Questions on Quiz
Question Details
Results
Date
Score
Hint
Time allowed
minutes
seconds
Time used
Answer Choice(s) Selected
Question Text
All done
Need more practice!
Keep trying!
Not bad!
Good work!
Perfect!